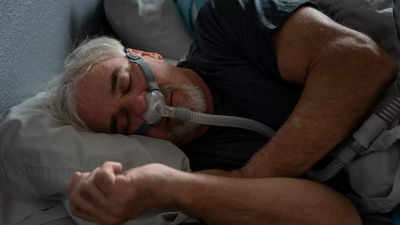
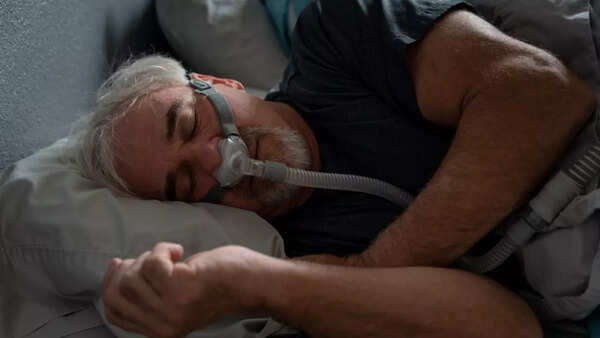
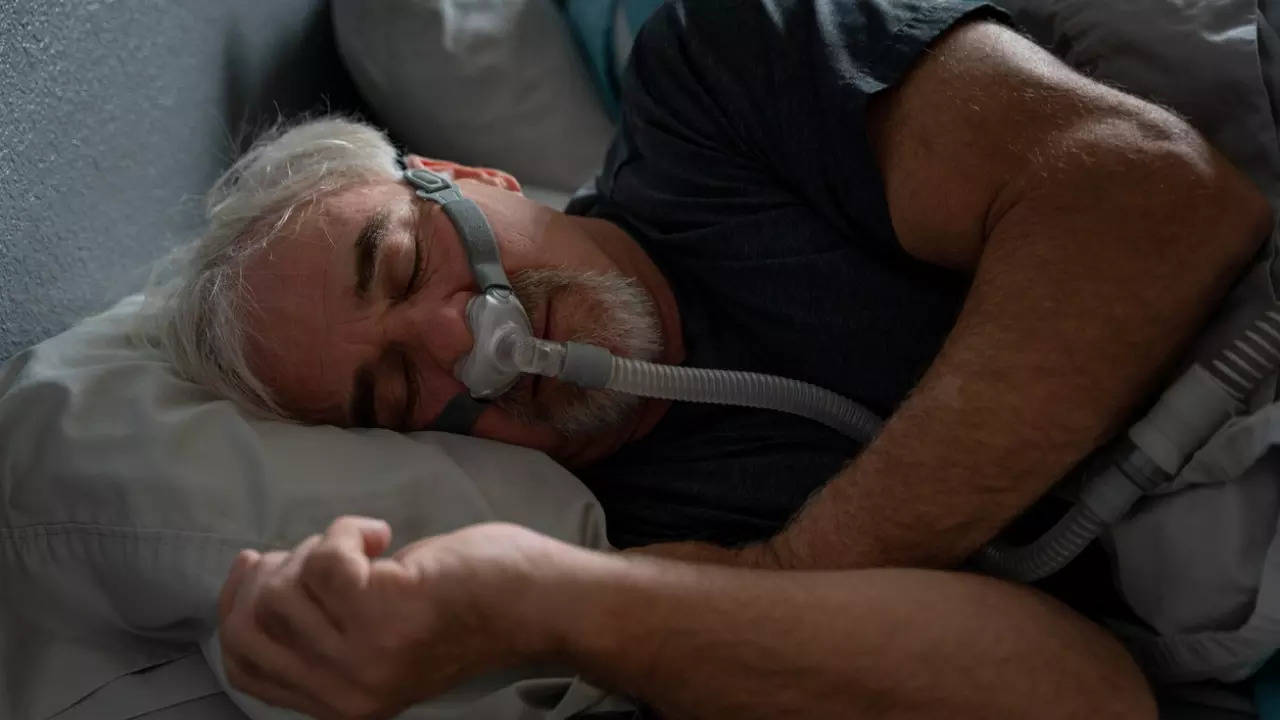
The FDA (Food and Drug Administration) has approved the first drug treatment for sleep apnea. permitting the use of a
weight-loss medication
for the condition that affects millions of Americans. On Friday, Dec. 20, the US regulator approved
Zepbound
(
tirzepatide
) for the treatment of moderate to severe
obstructive sleep apnea
(OSA) in adults with obesity, which promises results along with a calorie-deficit diet and increased physical activity.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea. This is a major step forward for patients with obstructive sleep apnea,” Sally Seymour, M.D., director of the Division of Pulmonology, Allergy, and Critical Care in the FDA’s Center for Drug Evaluation and Research said in a release.
What is obstructive sleep apnea?
Obstructive sleep apnea (OSA) is caused by episodes of airway collapse either partial or complete with an associated decrease in oxygen saturation or arousal from sleep. Though OSA can affect anyone, it is more common in people who are obese or overweight. The symptoms include loud, disruptive snoring, witnessed apneas during sleep, and excessive daytime sleepiness.
How does Zepbound work?
Zepbound activates the receptors of hormones secreted by the intestine, such as glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which reduces appetite and food intake. Studies suggest that by reducing body weight, Zepbound also improves OSA.
Zepbound is from the drugmaker
Eli Lilly
and Co, which is already approved for people who are obese or overweight and have a related health condition, such as type 2 diabetes, high cholesterol, or high blood pressure.
The drug makers conducted two randomised, double-blind, placebo-controlled studies in 469 adults without type 2 diabetes, for the approval of moderate to severe OSA in adults with obesity. One study included participants using positive airway pressure (PAP), the standard treatment for moderate to severe OSA, while the other involved those unable or unwilling to use PAP. The participants in both studies received either 10 or 15 milligrams of Zepbound or placebo once weekly for 52 weeks. After 52 weeks, those taking Zepbound showed a significant reduction in apnea and hypopnea events compared to the other group. Also, the participants treated with Zepbound had a significant decrease in body weight compared with placebo.
Side effects of Zepbound
- Nausea
- Diarrhea
- Vomiting
- Constipation, abdominal discomfort and pain
- Injection site reactions
- Fatigue
- Hypersensitivity (allergic) reactions (typically fever and rash),
- Burping
- Hair loss
- Gastroesophageal reflux disease.
The drug has also caused thyroid C-cell tumors in rats. Whether Zepbound would cause tumors, including medullary thyroid cancer, in humans is currently unknown.
People with these conditions should not take the drug
- Patients with a personal or family history of medullary thyroid cancer or patients with Multiple Endocrine Neoplasia syndrome type 2.
- Patients with a history of severe allergic reactions to tirzepatide (its active ingredient) or any of its other ingredients should avoid the drug.
- If a severe allergic reaction is suspected, patients should stop Zepbound immediately and seek medical help.
116539707 Warning
The drug also contains warnings for inflammation of the pancreas (pancreatitis), gallbladder problems, hypoglycemia (blood sugar that is too low), acute kidney injury, diabetic retinopathy (damage to the eye’s retina) in patients with type 2 diabetes mellitus, suicidal behavior or thinking, and pulmonary aspiration during general anesthesia or deep sedation.
- If you have symptoms of pancreatitis or gallstones, talk to your healthcare provider about it.
- Speak to your healthcare provider if you take insulin or medication that causes insulin secretion, to reduce the risk of hypoglycemia (low blood sugar).
- Health care providers are advised to monitor patients with kidney disease, diabetic retinopathy, depression, suicidal behaviors or thoughts.
- Patients taking Zepbound should inform healthcare providers of any planned surgeries of procedures.
(Pic courtesy: iStock)
I’m Manas Ranjan Sahoo: Founder of “Webtirety Software”. I’m a Full-time Software Professional and an aspiring entrepreneur, dedicated to growing this platform as large as possible. I love to Write Blogs on Software, Mobile applications, Web Technology, eCommerce, SEO, and about My experience with Life.